Glycyrrhiza uralensis (GU), a member of the Leguminosae family, is a traditional medicinal herb grown in various parts of the world . This plant has long been used to treat fever, liver ailments, dyspepsia, constipation, gastric ulcer, sore throat, asthma, and bronchitis . In addition, the roots have been extensively used for more than 4000 years as a sweetening and flavoring agent in candies, foods, tobacco products, and toothpastes. Flavonoids and triterpenoid saponins are shown to be the major constituents, which possess pharmacological activities . Among them, flavonoids, such as liquiritin and isoliquiritin, are gaining popularity because of their significant pharmacological activities, including antiulcer, antioxidant, anti-inflammatory, antimicrobial, antitumor effects, etc. . However, the free-radical-scavenging activities of phenolic compounds from the ethyl acetate extract of Glycyrrhiza uralensis have never been reported.
Dried roots from Glycyrrhiza uralensis (18 kg) were extracted with a 10-fold (w/v) volume of methanol by steeping for 3 days at room temperature three times and then filtered. The clarified solvent was evaporated under reduced pressure to afford the methanol extract (GUT; 3.50 kg). The extract was then suspended in distilled water (9000 mL) and partitioned successively with ethyl acetate (3000 mL × 4), and n-butanol (3000 mL × 4) to yield the ethyl acetate-soluble fraction (GUE; 823.7 g), the n-butanol-soluble fraction (GUB; 358.0 g), and the aqueous fraction (GUW; 2.3 kg). The GUE extracts showed better radical-scavenging activity than the other three extracts and were thus subjected to a series of chromatographic techniques such as silica gel (200–300 mesh) and Sephadex LH-20 column chromatography, PTLC, and prep-HPLC to yield compounds 1–14.
Bioassay-guided fractionation and purification of GUE from Glycyrrhiza uralensis afforded 14 phenolic compounds. These compounds were elucidated as licoricidin (1), 1-(3,4-dihydroxy-5-methoxyphenyl)-3-methylbut-2-ene (2) , semilicoisoflavone B (3) , platyisoflavanone B (4), naringenin (5) , glycyrin (6) , liquiritigenin (7) , gancaonin C (8) , glyasperins C (9) [9], glycycoumarin (10) , isoliquiritigenin (11) , 3,3′,4,4′-tetrahydroxy-2-methoxychalcone (12) , liquiritin (13) , and 6′′-O-acetylliquiritin (14) . Among the isolates, compounds 2 and 4 were isolated from the genus Glycyrrhiza for the first time.
To investigate the beneficial effects of Glycyrrhiza uralensis on oxidation,Wang Mingkui's group evaluated the antioxidant activity of the isolated compounds from Glycyrrhiza uralensis. The antioxidant capacity of the test samples (the extracts and phenolic compounds) were measured by their ability to scavenge the ABTS radical cation and DPPH radical using previously reported methods .
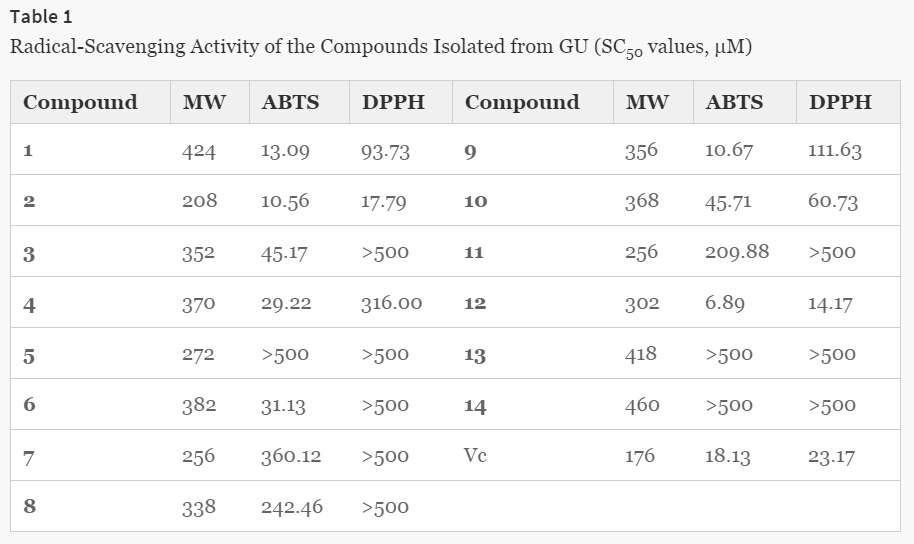
Fourteen compounds (1–14), were evaluated for their antioxidant activity by measuring their ability to scavenge ABTS and DPPH free radicals. The SC50 values are given in Table 1. Among all of the compounds tested, 1, 2, 9, and 12 exhibited potent ABTS radical-scavenging activity. Two compounds, 1-(3,4-dihydroxy-5-methoxyphenyl)-3-methylbut-2-ene (2) and tetrahydroxymethoxychalcone (12), showed very strong DPPH radical-scavenging activity. L-Ascorbic acid was used as a positive control. Based on their SC50 values, the ABTS radical-scavenging activity of the compounds in descending order was as follows: 12 > 2 > 9 > 1 > L-ascorbic acid (reference) > 4 > 6 > 3 > 10 >11 > 8 >7, while the descending order of the DPPH radical-scavenging activity of the compounds was as follows: 12 > 2 > L-ascorbic acid (reference) > 10 > 1 > 9 > 4.
The research has been published in Chemistry of Natural Compounds.