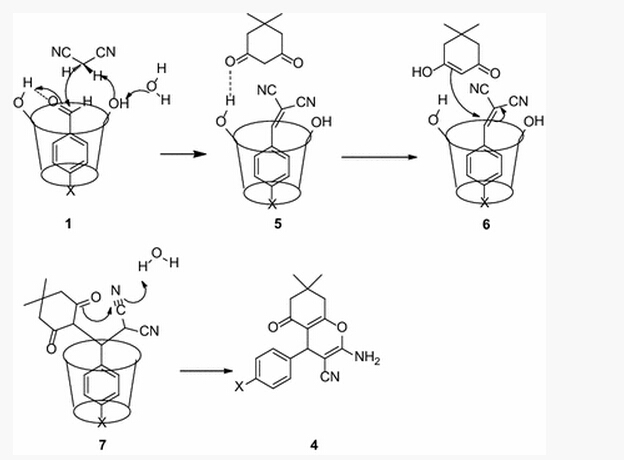
Synthesis of 4H-benzo[b]pyran and its derivatives has attracted great interest from organic and medicinal chemists, because of these products’ useful biological and pharmacological properties, such as spasmolytic, diuretic, anti-coagulant, anti-cancer, and anti-anaphylactic activities. 4H-pyrans is an important structural unit of a series of natural products. Additionally, some benzo[b]pyran derivatives have photochemical activities. Numerous synthetic methods have been reported for the synthesis of 4H-pyrans, involving use of a variety of catalysts such as TEBA, RE(PFO)3 , TBAF, TMAH, CeCl3 , Na2SeO4, Phenylboronic acid, DABCO, ZnO-beta zeolite, or the Ru(II) complex. However, these methods suffer from a variety of drawbacks, such as the use of hazardous and toxic solvents, high temperature, and difficult workup. Water is one of the most abundant, cheapest, and environmentally friendly solvents. However, most organic reactants are not soluble in aqueous media. Thus, an efficient phase transfer reagent is necessary to ensure the reaction is conducted in aqueous media. Recently, Azath et al. reported a new method to prepare 2-amino-4H-benzo[b]pyran, using per-6-amino-β-cyclodextrin under a solvent-free condition. Despite the difficulty in preparing the catalyst, organic solvent(s) were still needed in the product isolation step.
Wang Chun’ group describe a convenient, inexpensive and environmentally friendly method for the synthesis of 4H-benzo[b]pyran, using β-CD in water.
The research has been published in Research on Chemical Intermediates.