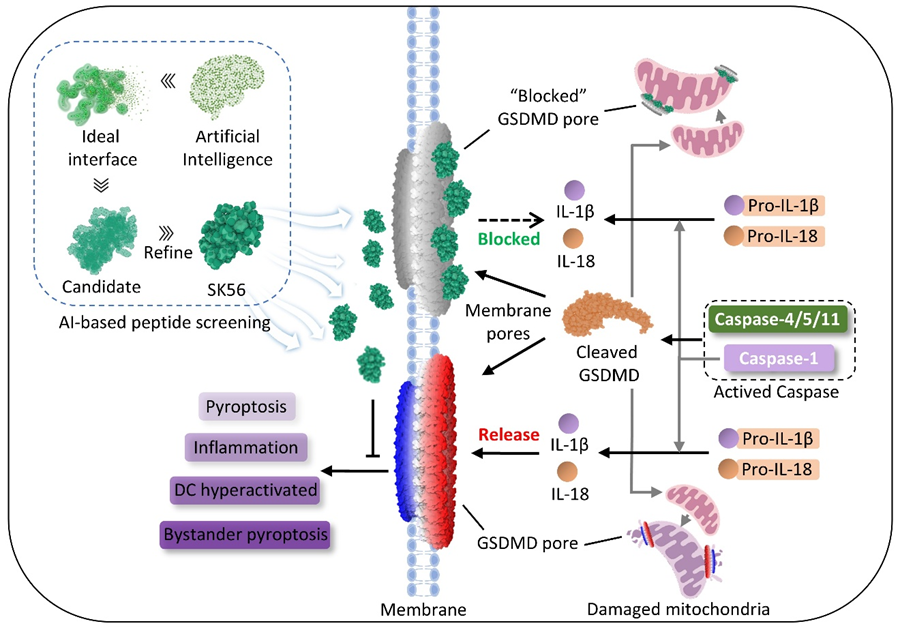
The pathogenesis of inflammatory diseases (such as sepsis and autoimmune disorders) is closely associated with overactive immune responses, in which gasdermin D (GSDMD)-mediated pyroptosis (a pro-inflammatory programmed cell death) plays a critical role. When activated, the N-terminal fragment of GSDMD (GSDMD-NT) forms pores on mitochondrial and cellular membranes, triggering pyroptosis and uncontrolled cytokine release. Recent studies have shown that extracellular vesicles can transfer mature GSDMD-NT pores to neighboring cells, thereby spreading the pyroptotic response—a phenomenon particularly prominent in sepsis patients. However, most current GSDMD inhibitors only target the pore-forming stage on membranes and are ineffective against already-formed pores, while sepsis patients typically receive treatment at this later stage. Therefore, there is an urgent need to develop inhibitors that can block established pores. However, designing such inhibitors is extremely challenging due to the large pore volume (approximately 21 nm in diameter), smooth surface, and highly dynamic structural changes. Additionally, complete inhibition of GSDMD function would affect its physiological roles, necessitating precise regulation rather than complete blockade.
To address this challenge, the research team employed a deep learning-based atomic generative model (TransForPep) to virtually screen peptide molecules capable of inhibiting mature GSDMD-NT pore function, validating candidate molecules in cell models, human alveolar lung organoids, and preclinical disease models. Through this approach, the team successfully identified the peptide molecule SK56, which selectively targets GSDMD-NT pores without affecting interleukin-1β processing or GSDMD activation.
Experimental results showed that SK56 effectively inhibits macrophage pyroptosis and cytokine release, and enters cells after pore formation to reduce mitochondrial damage. SK56 functions by relying on the cellular ESCRT membrane repair system and preventing dendritic cell overactivation and their uptake of GSDMD-NT membrane fragments from pyroptotic cells. In human alveolar organoid models, SK56 also prevented the spread of pyroptosis to neighboring cells. In delayed-treatment sepsis models, SK56 significantly improved mouse survival rates, even when administered after modeling had begun. This finding indicates that selective inhibition of GSDMD-NT pores by SK56 can block destructive inflammatory responses while preserving immune function.
SK56 provides a new candidate drug for the precise treatment of inflammation-driven diseases such as sepsis, chronic inflammation, and autoimmune disorders. Its ability to block pores after pyroptosis has initiated while protecting immune function has the potential to redefine treatment strategies for related diseases. Furthermore, this research demonstrates the potential of AI-guided peptide design in targeting "undruggable" biological structures, opening new avenues for biopharmaceutical development. Although the study data provide robust mechanistic insights, the experimental models do not fully replicate the complexity of natural infections, particularly systemic immune regulation environments. Future work will further explore SK56's effects in natural infection or trauma models, investigate its therapeutic window and synergy with existing anti-inflammatory drugs, and elucidate the structural basis of SK56-GSDMD interactions.
Our journey began with a fundamental question: Can we selectively limit pyroptosis without compromising immune defense functions to develop targeted therapies for inflammatory diseases? The discovery of SK56 exceeded expectations—it remains effective even after pyroptosis has begun, challenging the traditional belief that "pyroptosis is irreversible once triggered." This breakthrough not only expands SK56's therapeutic potential but also provides new insights for treating other inflammation-driven diseases. "Initially, we just wanted to find a 'switch to control inflammation,' but SK56's performance far exceeded expectations—it can even reverse existing damage," revealed a team member. "This makes us reconsider: perhaps humans can more intelligently 'coexist with inflammation' rather than simply suppressing it."
This study was published in Nature Immunology under the title "Delaying Pyroptosis With AI-Screened Gasdermin D Pore Blocker Mitigates Inflammatory Response." The multidisciplinary research was conducted by teams from the State Key Laboratory of Trauma and Chemical Poisoning, The Affiliated Yan’an Hospital of Kunming Medical University, Chengdu Institute of Biology/China-Croatia Belt and Road Joint Laboratory on Biodiversity and Ecosystem Services/Chinese Academy of Sciences, and Southwest University. Jianhui Sun and Jun Yang are co-first authors, while Dr. Gan Wang, Dr. Ping Meng and Dr. Ling Zeng are co-corresponding authors. The project was supported by the National Natural Science Foundation of China's Youth Science Program (B, C), General Program, Regional Joint Fund, National Defense Outstanding Youth Fund of the Bureau of Science, Technology and Industry for National Defense, and regional funds from Yunnan Province and Chongqing Municipality.
Original article: https://www.nature.com/articles/s41590-025-02280-x