Alkenyl iodides are highly valuable structural motifs and widely serve as important intermediates in the synthesis of various natural products, pharmaceuticals, and polymers. The iodide moiety is commonly regarded as the key element which provides simple and convenient avenues for further structural elaboration through transition-metal-catalyzed cross-coupling reactions, such as Heck, Suzuki, Stille, Sonogashira, and Negishi reactions.
In general, the strategies for the construction of alkenyl iodides are mostly based on iodination or hydroiodination of alkynes, allenes, propargylic alcohols, and their derivatives with iodinating reagents such as HI, I2, or N-iodosuccinimide. From a synthetic standpoint, iodoalkylation of alkynes could supply more versatile functionalized alkenyl iodides.
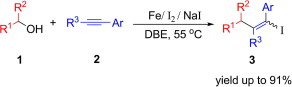
A new and efficient method for the synthesis of alkenyl iodides through direct coupling of alcohols and alkynes has been developed in the presence of iron powder, I2, and NaI. This methodology not only provides an attractive approach to alkenyl iodides, but also expands the application of iron in synthetic chemistry.
Prof.JI Jianxin's team from Chengdu Institute of Biology carried out this research, and were published in Tetrahedron Letters.