The optically active chiral thiols and sulfides are a key structural feature of several classes of pharmaceuticals and natural products and are extremely versatile building blocks that can undergo synthetically useful transformations,1 as well as have very important applications in asymmetric synthesis serving as ligands for metal-based catalysts,2 as catalysts themselves,3 and as chiral auxiliaries.
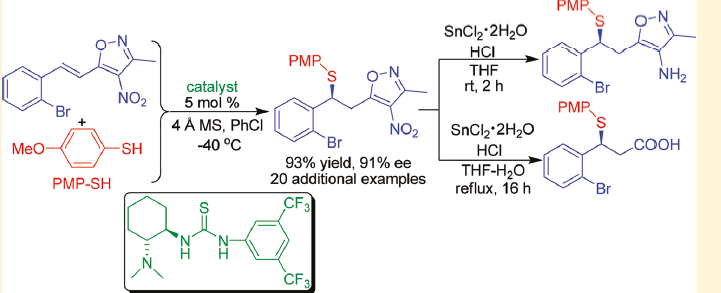
An enantioselective 1,6-Michael addition reaction of arylthiols to a wide range of 3-methyl-4-nitro-5-alkenyl-isoxazoles catalyzed by readily available Takemoto's thiourea catalyst has been developed. This reaction provides a useful catalytic method for the synthesis of optically active chiral sulfur compounds bearing a 4-nitroisoxazol-5-yl moiety in high to excellent yields (up to 97%) and high enantioselectivities (up to 91% ee). Significantly, the potential utilities of the protocol had been further demonstrated by gram-scale reaction and the versatile conversions of some resulting products into other functionalized and useful compounds,
Researchers from Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences and Chengdu Institute of Biology, Chinese Academy of Sciences cooperated to study this job and has published in JOURNAL OF ORGANIC CHEMISTRY in Oct of 2011. The job got grants from National Natural Science Foundation of China and National Basic Research Program of China (973 Program.